ANALYTICAL DEVELOPMENT SERVICES
Apeloa CDMO provides critical analytical method development and validation services for our contract customers. Whether it’s developing a unique test method for a new product or optimizing an existing test method for a new application, our experienced team of analytical scientists work closely with the applicable chemistry team to understand and fully characterize your molecule of interest. We also take pride in being efficient at developing methods that identify and even quantitate impurities in the product. Once a method has been established and optimized for the application, our team can execute a detailed validation protocol to document the robustness and compliance of the test method for use with the customer product to regulatory requirements.

Analytical method development is a critical step in the development of any product. Analytical methods allow scientists to identify, quantitate, measure, and characterize physico-chemical aspects of the molecule and related impurities. When compendial or literature analytical methods are not available, the importance of analytical method development expertise grows significantly.

EXPERIENCED ANALYTICAL DEVELOPMENT SCIENTISTS
Our analytical development teams are composed of highly educated and experienced pharmaceutical chemists and analysts. With extensive experience in the development and validation of analytical test methods, the Apeloa CDMO team has the knowledge to create validatable analytical methods for customer products.
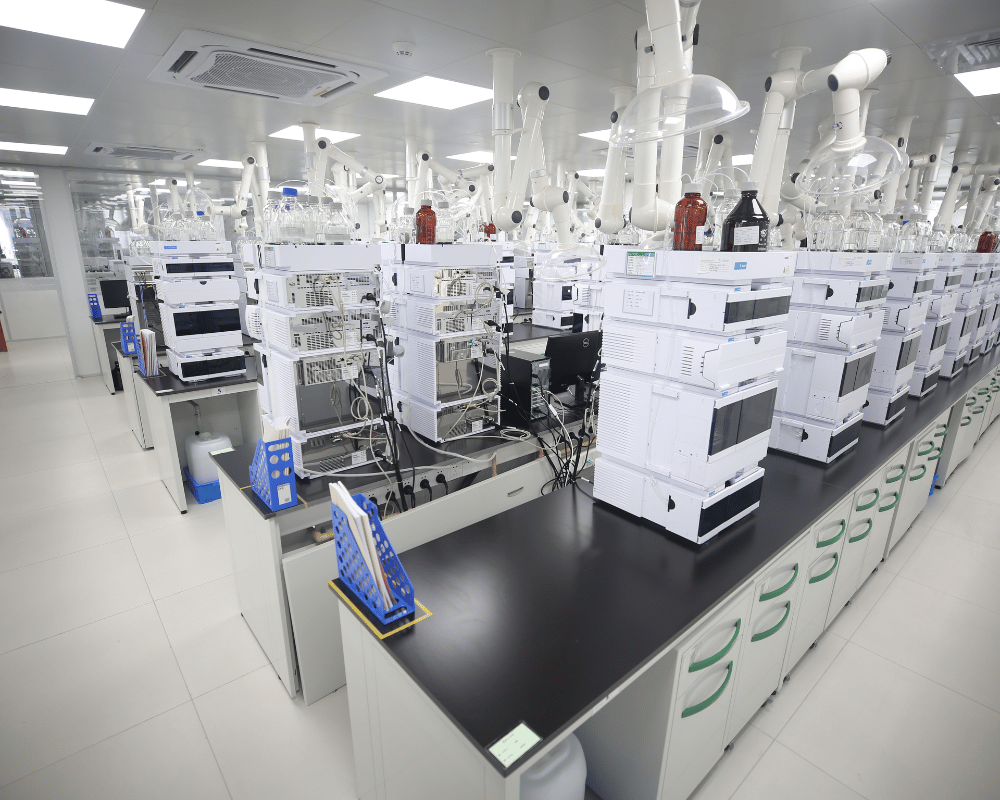
ANALYTICAL METHOD DEVELOPMENT & VALIDATION
Apeloa CDMO has built the experience and capabilities to develop robust analytical test methods for a wide range of molecules. Using a wide range of state-of-the-art analytical equipment, our laboratories can produce test methods that are appropriate for every product’s stage of development. Newly developed methods are optimized and can be quickly and comprehensively validated to current standards by our experienced analytical teams so you can have confidence in your product meeting the specifications defined on your Certificate of Analysis.

IMPURITY IDENTIFICATION
One critical aspect of analytical method development and testing of RSMs, Intermediates, and APIs is having the ability to identify unknown or undesirable impurities. Robust impurity analytical methods are crucial during the development process to optimize synthesis steps to reduce or eliminate unwanted impurities. Impurity methods are important for stability and release testing ensuring that the product is not generating impurities over time. Apeloa CDMO analytical development laboratories use a wide range of advanced instrumentation combined with decades of chemistry and analytical testing knowledge to identify the impurities and then measure and quantitate them in the product.

STATE-OF-THE-ART ANALYTICAL INSTRUMENTS
Apeloa CDMO manufacturing and development sites operate local laboratories capable of quickly and efficiently performing standard analytical testing and development services. We also have established centralized laboratory sites, such as our Apeloa Analytical Testing Center (AATC) that perform specialized testing. Samples are efficiently transferred to the central lab where they can be quickly tested to advance the development or manufacturing services being conducted. Some of the modern equipment utilized at our analytical laboratory sites that support our analytical development services include:
- X-Ray Diffractometer (XRD) – Rigaku Smart Lab SE
- Nuclear Magnetic Resonance (NMR) Advance NEO 600MHz
- Inductively Coupled Plasma Mass Spectrometer (ICP-MS) – Perkin Elmer NexION 1000G
- LC-Q-TOF Agilent 1290+ 6545
- Differential Scanning Calorimeter (DSC) – Mettler DSC3+
- Thermal Gravimetric Analysis (TGA) – Mettler TGA2
- LC-MSMS Shimadzu LC-400D + TQ8060NX
- LC-MSMS Shimadzu 30AD + TQ8060NX
- LC-MSMS Agilent 1290 +6470B
- LC-MSMS Waters XEVO TQ-XS
- GC-MSMS Agilent + 7000D
- GC-MSMS Shimadzu GC-2030 + TQ8050NX
- Micro Fourier Transform Infrared – FTIR
- High-Performance Liquid Chromatography (HPLC)
- Ultra-High-Performance Liquid Chromatography (UHPLC)
- Gas Chromatography (GC)
